Thermal Oxidation Process
Hiroyuki Kageshima and Masashi Uematsu
Physical Science Laboratory
Silicon thermal oxide films are essential elements in fabricating
silicon-based devices as well as even in future silicon devices. We have been
studying the unified understanding on the mechanism of the silicon thermal
oxidation process so as to control the process precisely in the atomic scale.
The oxidation process is generally thought to consist of two sequential processes;
the oxygen diffusion process through the covering oxide films and the oxygen
reaction process with the silicon substrate at the interface. However, we
think the oxygen reaction process should be further classified in two processes;
oxygen incorporating process with the substrate and the strain releasing process
by the structural transformation. Our picture is supported by recent isotope
experimental results, which show the back flow of some reduction species from
the interface into the oxide [1].
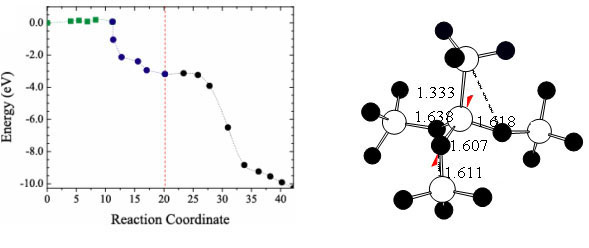
For the oxygen incorporation process, first principles studies have revealed
that there are not so high reaction barriers as expected by the general understandings
(Fig. 1) [2]. Thus, this process does not necessarily govern the interfacial
reaction speed. This is because the bonds of the oxygen molecule and of the
Si substrate do not completely break through the incorporation process.
For the strain releasing process, first principles studies have revealed
that the high-density oxide regions with oxygen vacancies (Fig. 2) formed
at the interface play important roles [3]. Even though these regions accompany
the oxygen vacancy, there is no broken dangling bond but only the covalent
bonds, being consistent with experimental quite low densities of electric
and magnetic defects. These regions can be regarded as regions with interstitial
SiO molecules, being consistent with the isotope experiments mentioned above.
[1] S. Fukatsu et al., Appl. Phys. Lett. 83 (2003) 3897.
[2] T. Akiyama and H. Kageshima, Surf. Sci. 576 (2005) L65.
[3] H. Kageshima et al., Jpn. J. Appl. Phys. 43 (2004) 8223.
 |
||
|
Fig. 1. Energy profile
in the oxygen incorporation process into the substrate |
Fig. 2. Structure of high-density oxide
regions with oxygen vacancies |
|