Fumihiko Maeda
Materials Science Laboratory
Single-walled carbon nanotubes (SWCNTs) are promising for the generation
beyond silicon-based microfabrication technology. However, the mixture
of various characteristics, such as metals and semiconductors with various
band gaps, are obtained for CNTs. The control of their characteristics
is essential in order to achieve large-scale integration for CNT-based
devices. Hence, we have been investigating the growth mechanism of CNTs
with the aim of controlling their growth and obtaining the desired CNTs.
Since catalytic nanoparticles play an important role in growing CNTs, their
chemical states are of great interest for this investigation. Recently,
we have succeeded in growing SWCNTs by CVD and performing successive in--situ x-ray
photoelectron spectroscopy measurements. As a result, we have been able to
elucidate the chemical state of catalytic nanoparticles after the growth [1, 2].
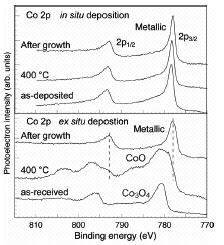
Figure 1 shows the core-level photoelectron spectra of Co, which is a
catalyst for the CNT growth. These spectra were captured before the growth
process, at the heating stage, and after CNT growth using ethanol. In the
case of ex-situ deposition, which is the conventional growth procedure, Co
was oxidized by exposure to air, resulting in the formation of Co3O4.
This oxide turned to CoO during the heating process under ultra-high vacuum
and, finally, metallic Co was obtained after CNT growth. In the case of in-situ deposition, the new process
without air-exposure after metal deposition, Co remained metallic throughout
the growth process. These two results indicate that the metallic state is
stable under the growth ambient. This is inconsistent with existing CNT growth
models, which need the carburization of catalytic nanoparticles. Meanwhile, the
CNT yield for the in-situ deposition
was higher than that for the ex-situ
deposition. From the analysis of core-level photoelectron intensity, we found
that the amount of decomposed carbon on the surface for the in-situ deposition is larger than that
for the ex-situ deposition. The
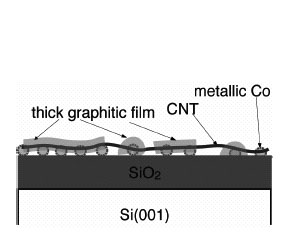
situation after CNT growth in the former case is schematically illustrated in
Fig. 2. The formation of thick graphitic films indicates that the uniform
metallic nanoparticles have a high ability for the decomposition and that a
large amount of migrating carbon remains without being included in the
nanoparticles during CVD. We will further investigate the reactions of CNT
growth and clarify the growth mechanism.
[1] F. Maeda, et al., Jpn. J. Appl. Phy. 46 (2007) L148.
[2] F. Maeda, et al., Mater. Res. Soc. Symp. Proc. 96 (2007) 30963-Q05-04.
 |
 |
||||
|
|