Superconductivity Hidden beneath Charge-Transfer Insulators
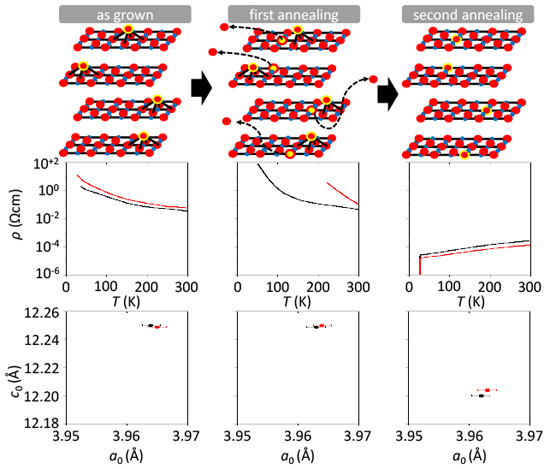
High temperature superconductivity (HTS) remains a mystery. For hole-doped cuprate superconductors doping is sufficient to induce superconductivity but such an approach does not hold for electron-doped cuprates. Moreover, while hole-doped cuprates are a build-up of CuO2 planes with copper being either five- or six-fold coordinated, electron doping only works when copper is four-fold coordinated. The fact that an annealing procedure is mandatory for the induction of superconductivity in cuprates with square-planar coordinated copper has obscured physicists for long while, hampering the development of effective theoretical models describing superconductivity in cuprates. Since the electron-doped side of the electronic phase diagram represents not only the quantity of doped electrons but to a much higher extent the influence of annealing, it is about time to liberate the phase diagram from these murky parameters. Quite in contrast to common belief (undoped cuprates are charge-transfer insulators), we have shown that electron doping does not represent an exigency but an option, i.e. superconductivity can be induced at any doping level, even zero, subject to an appropriate annealing treatment [1]. While the consequences of such elaborate annealing treatments for the induction of superconductivity are far reaching, the actual crystallographic changes within the crystal are subtle. Namely, as shown in Fig. 1, in Pr2CuO4, while the in-plane lattice constant remains constant (as-grown = 1st step = 2nd step) the c-axis length shrinks, particularly after the 2nd annealing step. Upon annealing an overstoichiometric oxygen at apical
sites [2] are being evacuated thus causing a reduction of the c-axis length.
Most importantly, the occupation of apical sites by oxygen have a dramatic
influence on the overall electronic- and magnetic properties as they are
the trigger for the insulator to metal transition in these systems. It
is the local binding energy of these extra oxygen ions that is influenced
by the amount of electron doping (Ce4+ substitution for Pr3+). For low Ce substitution levels, this bond strengthens and annealing conditions become delicate in order to avoid decomposition of the materials. Using a 2-step annealing scheme [3] may hold the key to effectively evacuate apical sites while keeping the crystal "alive".
- [1]
- Y. Krockenberger et al., Phy. Rev. B 85 (2012) 31.
- [2]
- P. G. Radaelli et al., Phy. Rev. B 49 (1994) 15322.
- [3]
- Y. Krockenberger et al., Sci. Rep. 3 (2013) 2235.
|
||
|
||