Michiya Fujiki
Materials Science Laboratory
Preparation of single-handed helical polymers is a long-standing issue
covering broad chemistries and practical applications, such as biochemistry,
polymer chemistry, supramolecular chemistry, analytical chemistry, and
chiral separation column and chiral pharmaceutical technologies. The widely
accepted way to selectively obtain a single helical polymer is to use an
enantiopure chiral substance with dextro- (d-) or levo- (l-) forms in residues,
repeating units, side chains, or initiators. Actually, for DNA, choice
of the corresponding d- or l-deoxyribose gives ideal mirror-image helical
motifs. Similarly, d- or l-amino acids provide the ideal mirror-images
of protein. Here we found that even enantiomerically impure chiral side-chains
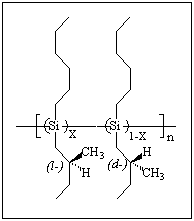
induce a left- or right-handed screw-sense helical structure of polysilane
copolymers consisting of d- and l- side-chains form.
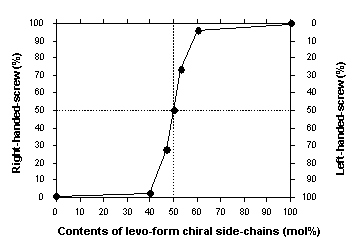
Figure 1 plots the contents of right-handed-helix as a function of
the contents of l-form chiral side-chains in the copolymers. Even
just 6 % enantiopurity (e. e.) of l-chiral side-chain induced an intense
right-handed helical form, while 6 % e. e. of d-chiral side-chain determined
its opposite-handed helical form. Interestingly, screw-pitch in the
racemic copolymers is independent of the purity of chiral side-chains because
the UV ?max peak in the copolymer systems was unchanged with the contents
of chiral side-chains in the copolymers. Although this might raise
the possibility of enantiomer-selective polymerization of racemic chiral
silanes, this would be excluded because the temperature dependence of the
UV and circular dichroism spectra of polymers with l-chiral / d-chiral
ratios = 60 / 40 (and 40 / 60) are different from those of enantiopure
l-chiral / d-chiral ratios =100 / 0 (and 0 / 100).
From the viewpoint of technological importance, the knowledge will
open a new way to easily obtain single-handed helical polymers from commercially
cheaper enantiomerically impure substances.
[1] M. Fujiki, Polym. Prepr. (Am. Chem. Soc. Polym. Sci. Div.) 37(1996)
454.
[2] Japanese Patent Pending.
[3] M. Fujiki, manuscript in preparation.


Fig. 1: Contents of right-handed-helix as a function of the contents of l-form chiral side-chains in the copolymers.