By fusing nanotechnology and biotechnology, we aim to fabricate a nanobiodevice that works with membrane proteins on a Si substrate. We succeeded in fabricating a microcavity array sealed with a lipid bilayer on a Si substrate for analyzing ion channel activity [1]. However, it is essential that we improve the lifetime of the nanobiodevice. In this study, we fabricated microcavities on a Si/SiO2 substrate covered by a thin SiO2 layer with nanohole arrays that we call a “pepper shaker substrate” [2].
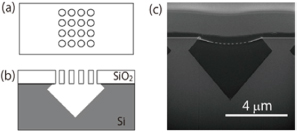
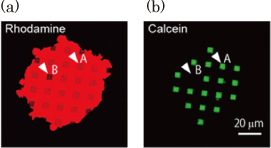
The nanohole arrays were fabricated in a thin SiO2 layer on a Si substrate using an electron beam lithography and dry etching technique. The Si substrate beneath the SiO2 overlayer was selectively etched through the nanohole arrays with a KOH aqueous solution, and this resulted in the formation of a microcavity covered by a thin SiO2 layer with a nanohole array as shown in Fig. 1. The microcavity volume was made comparable to that of a living neuron or synapse by choosing suitable etching conditions. The nanohole diameter (100 nm) was similar to the domain size of a cell membrane supported by cytoskeletons. The total area of the lipid membrane suspended over a nanohole array was kept large enough for the insertion of membrane proteins, although the area of a single nanohole was small. Figure 2 shows fluorescence images of a lipid membrane patch on a pepper shaker substrate. Calcein fluorescence [Fig. 2(b)] was observed at the microcavity where fluorescence from the lipid patch [Fig. 2(a)] was observed (for example, white arrow A). There were more than 100 nanoholes in each microcavity. If even one of the lipid membranes sealing the nanoholes were broken, fluorescent probes would flow out (white arrow B). Nevertheless, the small diameter of the nanoholes makes the suspended lipid membrane more stable, and almost all the microcavities were successfully sealed. This stabilization also increases the lifetime of the nanobiodevice. We confirmed that the green fluorescence was still confined in the microcavities after 10 days.
The stable sealing of the microcavities allows us to reconstitute and analyze membrane proteins embedded in suspended lipid membranes using fluorescence microscopy, AFM, and electrophysiology. We believe that this system could be employed for mimicking and studying the behavior of biological systems.