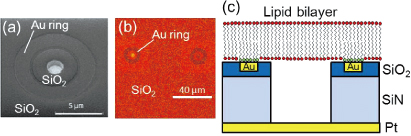
A sealed microwell with a lipid bilayer is a promising candidate for constructing artificial cells. If channel proteins can be incorporated into the membrane, their functional properties can be detected using electrophysiological or microscopic techniques. We have fabricated microwell structures sealed with lipid bilayers on a Si substrate, and succeeded in observing Ca2+ ion transport through α-hemolysin channels with fluorescent microscopy [1]. However, ion leakage through the water layer between the lipid bilayer and the substrate poses a problem when we try to achieve a low-noise biodevice. Here we present a newly designed microwell structure that uses a self-assembled monolayer (SAM) on a Au surface to prevent ion leakage from/into the microwells [2].
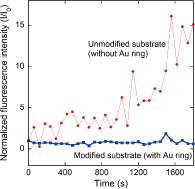
Figure 1 shows the device structure used in this study. We fabricated microwells with a slightly offset Au ring [Fig. 1(a)]. An octadecanethiol SAM was formed on the Au surface. The microwells were filled with calcein or Ca2+ indicators (fluo-4) and then sealed with lipid bilayers by rupturing giant unilamellar vesicles. Figure 1(b) shows a fluorescence image of a sealed microwell, in which calcein is confined. There is no fluorescence on the Au ring due to the energy transfer between the dyes and gold. Fluorescence recovery after photobleaching observation revealed that lateral fluidity is maintained across the Au ring. This result supports the structural geometry predicted in Fig. 1(c). Figure 2 shows the time course of the fluorescence intensity of fluo-4 confined in the microwells when CaCl2 solution was added to the outer solution. Unmodified substrates (without a Au ring) caused a significant increase in the fluorescence intensity within 20 min. However, with a modified substrate (with a Au ring), there was no increase in fluorescence intensity during the observation time. By separating the microwells and the outer regions, we can effectively reduce ion diffusion through the water layer. We estimated the membrane resistance with a view to realizing the electrophysiological detection of channel protein activity. The membrane resistances for the modified microwells were one order higher than those of the unmodified microwells, leading to a reduction in the background noise. The improved membrane resistance and background noise for the modified microwells resulted from the reduction in ion leakage. The device structure used in this study has the potential to provide a platform for measuring the very low signal-to-noise ratios of channel proteins.