Fabrication of a Hydrogel Array for Facilitating Lipid Membrane Formation
The integration of biological substances in electronic devices has great potential for various applications such as biosensing and drug discovery. Of the many kinds of biological molecules, membrane proteins have received a lot of attention due to their importance in relation to cellular survival and communication. We have already developed nano-bio devices for the optical and electrophysiological analysis of membrane proteins by using microwells on a silicon substrate covered with lipid bilayers [1]. In addition, we have improved the fragility of a suspended lipid membrane by using hydrogel as a water permeable and mechanical support [2].
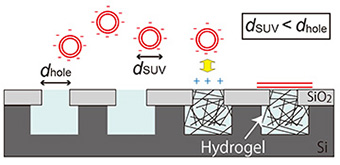
Here, we describe the fabrication of a cationic hydrogel array that can electrostatically facilitate the formation of a planar lipid membrane over microwells by the fusion of negatively charged small unilamellar vesicles (SUVs) with a diameter smaller than the microwell aperture (Fig. 1) [3].
The hydrogel array was produced as follows: microwells 1, 2, 4, and 8 μm in diameter and
1 μm deep were fabricated on a silicon substrate using a conventional lithographic and etching technique. The hydrogel was prepared by photo-initiating free radical polymerization from a cationic gel-precursor aqueous solution including a green fluorescent dye, calcein. The hydrogel precursor solution was dropped onto the substrate and polymerized by UV light irradiation through a photomask covering half the area of the substrate. After removing excess hydrogel from the substrate, a lipid membrane was prepared on the hydrogel-confined microwell by rupturing negatively charged SUVs with a diameter smaller than the microwell aperture. The lipid membrane formation on the hydrogel-confined microwell array was observed with fluorescence microscopy (Fig. 2).
The positively charged hydrogels were only confined in the microwells on the substrate where light was irradiated [Fig. 2(a)]. Planar lipid membranes were formed on the microwells confining the positively charged hydrogels [Fig. 2(b)]. By comparison, lipid membranes were not formed in microwells with no positively charged hydrogels [Fig. 2(c)]. These results indicate that the function of the hydrogels confined in the microwells is to both stabilize the lipid membranes mechanically and facilitate the formation of a lipid membrane via an attractive electrostatic interaction between SUVs and hydrogels. We expect these hydrogel supports to allow us to prepare a lipid membrane from a proteoliposome. Since the chemical composition of a hydrogel is easily modified, we can obtain a hydrogel array with desirable properties such as high mechanical strength, electric charge, and responsiveness to a stimulus.
- [1] K. Sumitomo et al., Biosens. Bioelectron. 31, 445 (2012).
- [2] A. Tanaka et al., Appl. Phys. Express 7, 017001 (2014).
- [3] A. Tanaka et al., Colloids Surf. A 477, 63 (2015).
 |
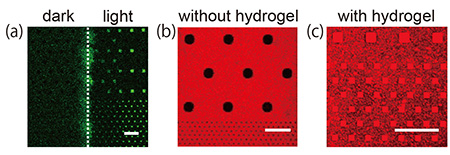 |
| Fig. 1. Schematic illustration of lipid membrane formation by fusing SUVs on a hydrogel array. | Fig. 2. (a) Fluorescence image of a substrate after light irradiation over half the substrate area. (b, c) Lipid membrane formation on the substrate (b) without and (c) with light exposure. Scale bar: 20 μm |