Kazuhide Inoue2, and Keiichi Torimitsu1
1Materials Science Laboratory, 2Kyushu University, 3Yamanashi University
Proteins, known as "receptors" that exist in cells, exhibit
their functions via binding with small compounds such as hormones. When
the receptor is a type of ion channel, it opens its pore after binding
with compounds thereby flowing ions through it and resulting in exhibiting
its function. We observed the topology and stimulation-induced conformational
changes in adenosine triphosphate (ATP) receptor [1], an important receptor
in pain sensation, with atomic force microscopy (AFM).
ATP receptor gene was over-expressed in immortalized cells. ATP receptor
proteins were purified from their cell membranes and receptors for AFM
observation were adsorbed on fleshly cleaved mica. Non-stimulated ATP receptor
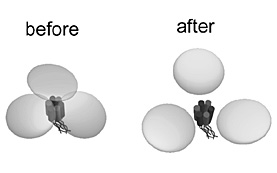
exhibited circular feature and that after ATP stimulation was in tripartite
morphology (Fig. 1). To study whether structural difference between these
two state is derived from the stimulation-induced conformational change
in ATP receptor, we performed time-lapse imaging of conformational changes
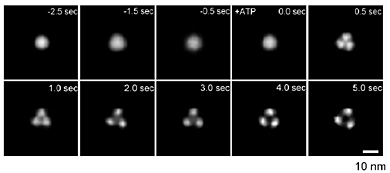
in ATP receptor with fast-scanning AFM. Before stimulus, ATP receptor exhibited
circular feature (Fig. 2, -2.5〜0.0 s). After stimulus, ATP receptor immediately
changed its structure into trimeric topology (Fig. 2, 0.5 s). Detailed
analysis of trimeric ATP receptor revealed that it exhibited the further
disengagement of three subunits and large pore-like structure in its center
(Fig. 2, 2.0〜5.0 s). To confirm whether these structural changes are related
to the physiological functions, we measured permeability of ATP receptor
channel using fluorescent molecules. ATP receptor exhibited permeability
to calcium ions and appeared to be functional. When imaging buffer contains
no calcium, ATP receptor exhibited permeability to larger molecules (ethidium
bromide) but not when imaging buffer contains calcium. These results indicate
that structural changes in ATP receptor appeared to correspond to the physiological
function via flowing ions through its pore. Thus, we succeeded in observation
of topology and structural changes related to physiological functions of
ATP receptor. We will reconstitute receptors into artificial lipid bilayer
on a flat substrate [2] and analyze the relationship of receptor-lipid
interaction and receptor topology/function.
[1] Y. Shinozaki et al., PLoS Biol. (accepted).
[2] Y. Shinozaki et al., Jpn. J. Appl. Phys. 47 (2008) 6164.
 |
 |
|||||
|
|