Materials Science Laboratory
Both aluminum nitride (AlN) and diamond have wide bandgaps: 6.0 eV for
AlN and 5.5 eV for diamond. On the other hand, they have opposite doping
characteristics. For AlN, n-type doping is easier than p-type doping, while
for diamond, p-type doping is easier than n-type doping. The AlN/diamond
heterostructure is expected to combine the features of both materials and
appears promising for achieving high-efficiency deep-ultraviolet light-emitting
diodes and high-power electron devices. The key to realizing these devices
is single-crystal growth of AlN on diamond. However, to date, due to the
difference in the crystal structures (hexagonal structure for AlN; cubic
structure for diamond), AlN layers grown on diamond substrates have had
multi-domain structures [1].
Here, using diamond (111) plane, we grew the AlN layer on the diamond
substrate because the atomic bonding configuration of the hexagonal AlN
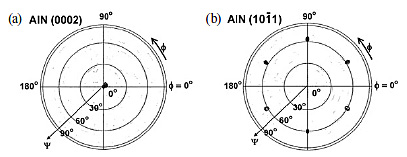
(0001) plane is similar to that of the cubic diamond (111) plane. Figure
1 shows the X-ray pole figures of AlN (0002) and (10
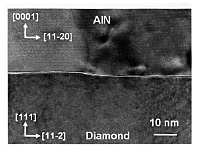
In the cross-section TEM image at the heterointerface (Fig. 2), an abrupt AlN/diamond interface was observed without any interface layer. This confirms that the single-crystal AlN (0001) layer epitaxially grows from the nucleation step just on the diamond (111) surface. At the AlN(0001)/diamond(111) interface, there are two possible bonds, C-N or C-Al bonds. Because the bond energy of the C-N bond (3.1 eV/bond) is stronger than that of the C-Al bond (2.6 eV/bond), the C-N bond is preferentially formed at the AlN/diamond interface. As a result, the AlN layer grown on diamond has Al polarity.
For the AlN(0001)/diamond(111) heterostructure, the in-plane epitaxial
relationship is [10
[1] K. Hirama, Y. Taniyasu, and M. Kasu, Jpn. J. Appl. Phys. 49 (2010) 04DH01.
[2] Y. Taniyasu and M. Kasu, J. Cryst. Growth 311 (2009) 2825.
 |
 |
|||||
|
|