Materials Science Laboratory, *Osaka University
We have succeeded in growing carbon nanotubes (CNTs) from diamond. CNTs
are promising as future base components in many industrial fields due to
their various superior properties, such as low weight, high mechanical
strength, and good electrical/thermal conductivities. So far, Fe, rare
metals like Co and Ni, and noble metals like Au, Ag, and Pt have been used
as effective catalysts for CNT synthesis. However, because these metal
catalyst particles easily lose their catalytic activities due to aggregation/fusion
and reaction with substrates, they are not suitable for high-density CNT
growth. Furthermore, because these metal catalyst particles are in a liquid
phase at the single-wall CNT (SWCNT) growth temperatures, they cannot be
used to precisely control the CNT structure, especially the chirality.
Diamond is free from aggregation/fusion and is in a solid phase at the
growth temperatures. Therefore, we hope diamond will make it possible to
grow CNTs with a precisely controlled structure.
In this work, we clarified that the CNT syntehsis from diamond by chemical
vapor deposition (CVD) requires (1) the use of nanodiamond particles with
a diameter smaller than 5 nm [1]; (2) removal of the graphite formed on
the nanoparticle surface; and (3) the use of gases that thermally decomposed
easily, such as ethanol vapor and acetylene, as the carbon feed stock [2].
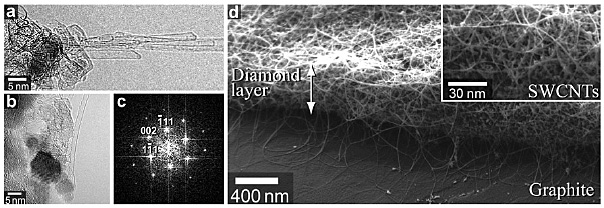
Transmission electron microscopy (TEM) images [Figs. 1(a) and 1(b)] and
the Fourier transform pattern [Fig. 1(c)] obtained from Fig. 1(b) indicate
that SWCNTs are grown from nanodiamond particles. Scanning electron microscopy
(SEM) image in Fig. 1(d) indicates that SWCNTs can be grown from three-dimensionally
accumulated nanodiamond particles. This means that the nanodiamond particles
were in the solid phase at the CVD ambient conditions and did not aggregate.
It is known that, in metal-catalyzed CNT growth, carbon atoms are supplied
to the CNT through bulk diffusion of carbon atoms in the particle. On the
other hand, CNT growth from nanodiamond particles must be promoted by surface
diffusion of carbon atoms.
Nanodiamond particles are produced at low cost and have catalyst activities
as high as metal nanoparticles. The nanodiamond particles offer the possibility
of not only low-cost, high-density growth of SWCNT but also precise control
of the structure of SWCNT.
[1] E.
[2] D. Takagi et al., J. Am. Chem. Soc. 131 (2009) 6922.
 |
||
|