Membranes: Functional and Structural Properties
University of Oxford, *Materials Science Laboratory
Alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs) are glutamate-gated ion channels ubiquitous in the central nervous system where they mediate fast excitatory neurotransmission, and act as molecular determinants of memory formation and learning. Structural studies of full-length AMPARs by electron microscopy (EM) and X-ray crystallography have provided important insights into channel assembly and function. However, the correlation between structure and functional states of the channel remains ambiguous, particularly since these functional states can only be assessed with the receptor bound within an intact lipid bilayer. Our group demonstrated structural examination of reconstituted functioning GluA3 by using atomic force microscopy (AFM) [1], however, the reason why the observed protein height was smaller than that observed by EM was still unknown.
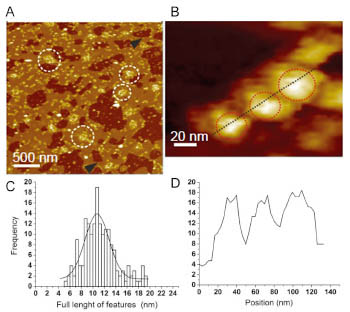
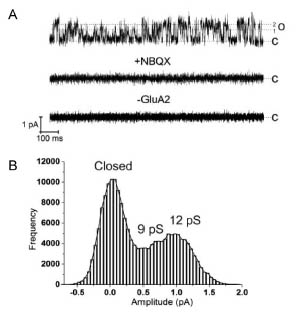
In this study, to provide a basis for investigating AMPAR structure in a membrane environment, we developed an optimized reconstitution protocol of GluR2 whose structure has previously been characterized by EM, and examined their function and structure [2]. AFM studies of the reconstituted samples provide high-resolution images of full-length membrane-embedded AMPARs at densities comparable to those in postsynaptic membranes (Fig.1). Single-channel recordings of reconstituted GluA2 recapitulate key electrophysiological parameters of the channels expressed in native cellular membranes (Fig.2). The data demonstrate the effect of protein density on conformational flexibility and dimensions of the receptors and provide the first structural characterization of functional, membrane-embedded AMPARs, thus, laying the foundation for correlated structure-function analyses of the predominant mediators of excitatory synaptic signals in the brain.
[1] N. Kasai et al., BBA General Subjects 1800 (2010) 655.
[2] J. Baranovic, C. S. Ramanujan, and N. Kasai, J. Biol. Chem. 288 (2013) 8647.
 |
 |
|||||
|
|