Stable Sealing of Microcavities with a Lipid Membrane for Nanobiodevices
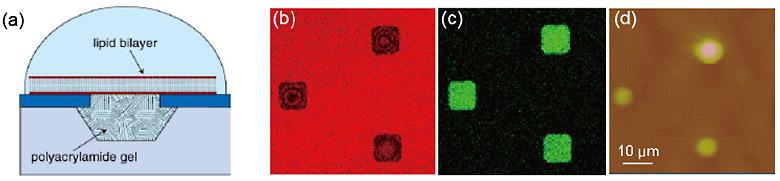
The integration of biological substances in electronic devices has great potential for various applications such as biosensing and drug discovery. We have developed nanobiodevices for the optical and electrophysiological analysis of biomaterials, especially membrane peptides and proteins, by using microwells on a silicon substrate covered with lipid bilayers [1]. However, a lipid membrane suspended over a microwell is unstable, so it is difficult to measure the membrane protein function over a long period. It is known that the cell membrane in living cells is stabilized by proteins anchored to the cell membrane and the cytoskeleton. The fabrication of a cytoskeleton-mimetic structure in microwells promises the mechanical improvement of the device. Here, we describe a hydrogel confined in a microwell array as a potential cytoskeleton candidate for the mechanical support of lipid bilayers (Fig. 1(a)) [2].
The hydrogel array was produced as follows: microwells 1, 2, 4, and 8 µm in diameter and 1 µm deep were fabricated on a silicon substrate. The hydrogel was prepared from an aqueous solution of hydrogel precursors including calcein, which yields a green fluorescence on the substrate. Before the polymerization was complete, the microwells were sealed with lipid bilayers by rupturing giant unilamellar vesicles including rhodamine with red fluorescent emission on the substrate. The hydrogel formed out of the wells was removed by pushing it in a direction horizontal to the substrate.
The formation of lipid bilayers on the hydrogel-confined microwells was observed with fluorescence microscopy and atomic force microscopy (AFM). Fluorescence images are shown in Fig. 1(b) and (c). Red florescence from the rhodamine in the lipid bilayers was observed at the microwells where calcein fluorescence was observed in the hydrogel solution. Microwells filled with hydrogel were observed by AFM (Fig. 1(d)). These observations indicate that the lipid bilayer confines the hydrogel precursors in the microwells and the hydrogel supports the formation of a lipid bilayer for at least 2 weeks.
Since the chemical composition of a hydrogel is easily modified, we can obtain hydrogels with desirable properties such as mechanical strength, an electric charge, and responsiveness to a stimulus. The array has potential applications to the functional reconstitution of living cells in microwells.
- [1]
- K. Sumitomo et al., Biosensors and Bioelectronics 31 (2012) 445.
- [2]
- A. Tanaka, H. Nakashima, Y. Kashimura et al., Jpn. J. Appl. Phys., 53 (2014) 01AF02.
|
|
|
|