Ligand-Induced Structural Changes in a Single Ion Channel Receptor
We have attempted to fabricate "nano-biodevices" for detecting and regulating biological functions. In this study we investigated ligand-induced structural changes in ion channels reconstituted into a supported membrane on a mica substrate using fast-scanning atomic force microscopy (AFM) [1]. Fast-scanning AFM enables us to visualize the structure of a single protein with sub-nanometer scale resolution at up to 80 ms/frame, which is ideal for the structural examination of biological molecules such as proteins.
We employed an N-methyl-D-aspartate type ligand-gated ionotropic glutamate receptor (GluNR) obtained from rat cortical neurons and used an electrophysiological method to reveal that native GluNRs in the suspended membrane exhibited ion channel activity.
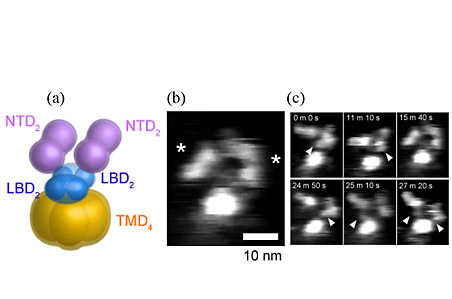
On the basis of recent studies of GluNRs, it is suggested that ligand binding domains (LBDs) and N-terminal domains (NTDs) have two-fold symmetry and a dimer-of-dimers structure
(Fig. 1(a)). When we imaged membrane-unreconstituted GluNRs, they exhibited a characteristic structure with two saddle-shaped particles (corresponding to dimeric NTDs) and a circular particle (corresponding to a transmembrane domain (TMD)) (Fig. 1(b)). Long-term imaging of a single GluNR channel has shown the structural flexibility of NTDs (Fig. 1(c)).
We then investigated ligand-induced structural changes in the extracellular
part of GluNRs reconstituted in supported membranes quantitatively (Fig.
2). Without ligands, the tetrameric particles of NTDs exhibited various
conformations including a circular structure without subunits, structures
with two- and four-fold symmetry, and a dimer-of-dimers structure. After
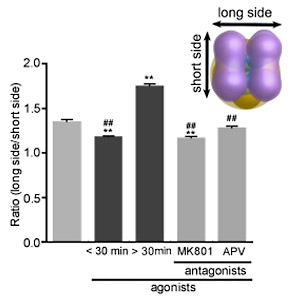
being treated with an agonist, the NTDs appeared to be less flexible, and
many of them were in the dimer-of-dimers conformation. After a prolonged
agonist treatment (> 30 minutes), a large gap was formed between the
two NTD dimers, which were suppressed by pre-treatment with GluNR antagonists.
These results suggested that the extracellular parts of the ion channel receptors were highly flexible and dynamic, and demonstrated that their structure can be controlled by using exogenously applied small compounds even after they have been reconstituted into supported lipid bilayers on a Si substrate.
- [1]
- Y. Shinozaki, A. Tanaka, N. Kasai, et al., Appl. Phys. Express 7 (2014) 027001.
 |
 |
|
|