On-chip FRET Graphene Oxide Aptasensor: Enhanced Sensitivity by Using Aptamer with Double-stranded DNA Spacer
Graphene and graphene oxide (GO) behave as efficient acceptors for energy transfer between a graphene/GO surface and molecules located close to it. By combining the energy transfer reaction with a biomolecule reaction, we can visualize an invisible biological response to a measurable physical quantity such as fluorescence.
We have successfully demonstrated a unique type of fluorescence biosensor, namely a graphene/GO aptasensor, for selective and highly sensitive protein detection. We realized this sensor by modifying the graphene/GO surface with a pyrene-aptamer-dye probe that we developed (biomolecular interface). These three components of the probe work as a linker to the GO surface, a protein recognition part, and a fluorescence detection tag, respectively [1]. The system allows us to perform molecular detection on a solid surface, which is a powerful tool for realizing an on-chip sensor, and especially for forming a multichannel configuration and for micropatterning probes [2]. The on-chip sensor allows us to evaluate the sensor response quantitatively by using one of the channels/patterns as an internal standard.
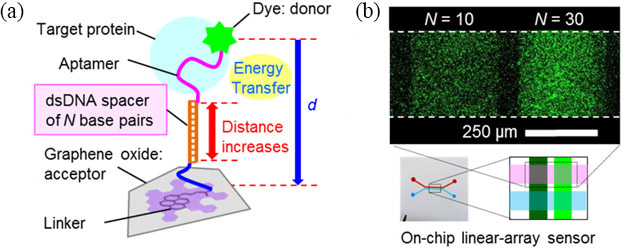
The most attractive feature of aptamers is that they can be flexibly designed without loss of activity. We designed biomolecular probes for highly sensitive protein detection by modifying an aptamer with (i) a single-stranded DNA spacer between the aptamer sequence and the dye [3] and (ii) a double-stranded DNA spacer between the aptamer sequence and the graphene/GO [Fig. 1(Left)] [4]. The spacer controls the distance between the dye and the graphene/GO, which is crucial for the energy transfer reaction (FRET, fluorescence resonance energy transfer). We improved the sensitivity of an on-chip GO aptasensor by using a longer spacer for probes (i) and (ii) [Fig. 1(Right)]. By using the best probe design, we achieved a detection limit of ~1 nM for thrombin, which is in the in vivo concentration range during blood clotting [3].
- [1] K. Furukawa et al., J. Mater. Chem. B, 1, 1119 (2013).
- [2] Y. Ueno et al., Anal. Chim. Acta, 866, 1 (2015): Featured on cover.
- [3] Y. Ueno et al., Chem. Commun., 49, 10346 (2013): Featured on cover.
- [4] Y. Ueno et al., Anal. Sci., 31, 875 (2015): Hot Article Award.
|
||
| Fig. 1. (a) Molecular design of the probe (ii) for enhancing sensitivity of the aptasensor. (b) Quantitative comparison of enhancement effect depending of the spacer length. | ||