@
Kazuaki Furukawa
Materials Science Laboratory
@
@Self-spreading is a lipid wetting process at a solid-liquid interface,
where a single supported lipid bilayer (SLB) is formed by a self-assembly
process at the rim of a lipid spot adhering to a hydrophilic surface. We
examined the self-spreading on the patterned surface and found that self-spreading
occurs only on the hydrophilic surface of SiO2. This led us to propose a
new type of microchannel device, which we call "lipid-flow chip" [1].
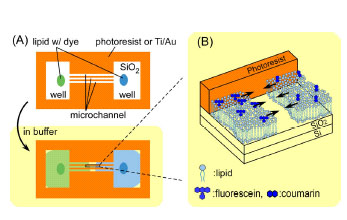
@The device structure and principle of the device operation are shown
schematically in Fig. 1. The device is equipped with microchannel part
of 10 Êm wide and 400 Êm long, and has a well on each side, which has
been fabricated by conventional photolithography and lift-off process.
A single self-spreading SLB grows only on hydrophilic surfaces after the
SLB has been introduced into the pattern. When two SLBs collide in the
middle of the channel, they form a unified SLB where molecular diffusion
from one side to the other becomes dominant. SLBs are used only for transporting
the molecules of interest. Thus an SLB is regarded as, for instance, a
carrier gas for gas chromatography.
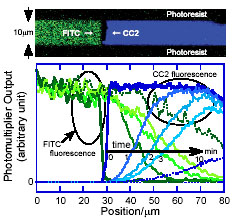
@The device is beneficial for detecting an intermolecular interaction.
As one example, we demonstrate the observation of fluorescence resonance
energy transfer (FRET) between a donor (CC2) and an acceptor (FITC). As
shown in Fig. 2, two lipid bilayers containing each dye-conjugated molecule
was collided with each other in the microchannel. After the collision,
they form a unified lipid bilayer, and the dyes are mixed with each other
by lateral diffusion. The distribution of dye concentration is symmetrical
to the point of the collision. A great reduction in donor fluorescence
is, however, observed in a mixed area of donor and acceptor. This is because
of the two relaxation processes, emission and FRET, exists in excited donor.
The great reduction of donor emission is attributed to the effective FRET.
Our device is also advantageous for quantitative analyses of FRET efficiency
between a variety of dye pairs.
@We plan to apply the technique to enzymes and proteins in order to study
molecular interactions such biomolecules have.
[1] K. Furukawa, et al., Lab Chip 6 (2006) 1001.
@
 |
@ |  |
||||
|
@ |
|