Goo-Hwan Jeong, Satoru Suzuki and Yoshihiro Kobayashi
Material Science Laboratory
The electrical properties of carbon nanotubes (CNT) are highly dependent on their geometric structures, such as their diameter or chirality. Thus, for the application of CNT-based nanoelectronics, it is essential to develop growth methods in which diameter or chirality can be controlled. Here, we introduce our recent work for diameter-controlled CNT growth, in which we used catalytic nanoparticles instead of a thin-film catalyst [1].
As the catalyst source, we used ferritins, which are covered with protein shell and contain Fe (ferritin) or Co clusters (Co-ferritin) in their core (6-8 nm in diameter). We also used Dps protein, which contains Fe clusters in its core (4-nm in diameter). Catalytic nanoparticles were obtained by calcination of ferritin-family molecules. We grew CNTs on flat or pillar-patterned substrates by means of ferritin-casting, calcination, and successive methane CVD.
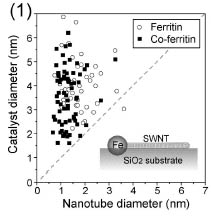
Figure 1 shows a result of atomic force microscopy (AFM) measured after
CNT growth using ferritins and Co-ferritins [2]. Considering that the nanoparticles
are 2-7 times larger than the CNT diameter, we can suppose that thin CNTs
grow from the large catalytic nanoparticles. The inset is schematic illustration
of the base-growth mechanism. Fe-catalyst embedment in SiO2 substrates was confirmed by transmission electron microscopy.
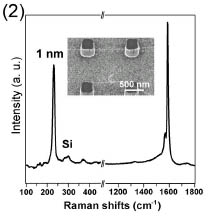
The Raman profile and a scanning electron microscopy (SEM) image of a suspended CNT (inset) grown from Dps are shown in Fig. 2. From the Raman spectra, we know that the CNT diameter is about 1 nm and that structural quality of the CNT is very high.
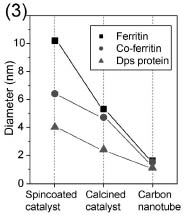
The results of size changes of ferritin-family catalysts and resultant CNT diameters are summarized in Fig. 3. The ferritin-family molecules (10.2-4.0 nm) are changed to catalytic nanoparticles (5.3-2.4 nm) by calcination and finally give rise to thin CNT (1.6-1.1 nm). We found that the diameter differences between catalysts and CNT become smaller as the catalyst size decreases. Further, the CNT diameter itself becomes smaller. These suggest that smaller catalysts than those used in the present work will provide critical clues for diameter control of CNTs. Investigating not only the catalyst behavior during CVD growth [3] but also the size-relationship between catalysts and CNT is critical for diameter-controlled CNT growth.
[1] G. H. Jeong et al., J. Am. Chem. Soc. 127 (2005) 8238.
[2] G. H. Jeong et al., J. Appl. Phys. 98 (2005) 124311.
[3] G. H. Jeong et al., Chem. Phys. Lett. 422 (2006) 83.
 |
 |
 |
||||||
|
|
|