Success in time-lapse imaging of conformational changes in a single receptor protein
- Proposal of a new model of conformational changes in ATP receptor protein -
NTT Basic Research Laboratories, in collaboration with Kyushu University, has succeeded in observing a structure and its dynamical changes using a single receptor protein, “ATP receptor”. The observations were performed under conditions that allow the protein active as it were in the cell, using fast-scan atomic force microscopy (AFM) that has nanometer scale resolution.
The neural network in brain is responsible for signal transmission and signal processing in life. The key molecule for the signal transmission is receptor protein. Researchers believe that the receptor protein transmits signals by changing its structures when the specific molecule binds; the structural changes of the proteins during the operation, however, are not fully understood. We have succeeded in observing dynamic changes of ATP receptor protein structure when ATP binds to the protein and proposed a new model to explain the mechanism of structural changes when ATP receptor functions. The result was achieved by combining the state-of-the-art nanotechnology in NTT and the advanced biotechnology in Kyushu University.
Our techniques can be applied to many other receptor proteins and is useful for elucidating their structures and physiological functions. An understanding of the biological signal transduction mechanism will help us to fabricate new conceptual information and communication technology that mimics biological mechanisms. Moreover, by using these results and techniques, we hope to fabricate new devices that combine biological molecules and electric devices.
The result will be published in PLoS Biology online on May 5th, 2009 (PST). This study was supported by JST Strategic International Cooperative Program (SICP), Japan and United Kingdom and Japan Health Science Foundation, Health, Labor and Welfare Ministry Grand-in-Aid for Scientific Research.
=> Molecular and Bio Science Research Group
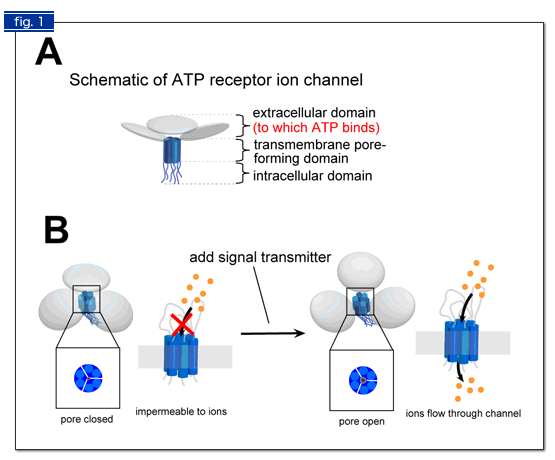
Fig. 1. Schematic illustrations of ATP receptor structure (A) and how it performs its function (B).
(A) An ATP receptor exists on cell membrane. A single ATP receptor is formed by three subunits. The subunit possesses three domains: an extracellular domain (shown in light blue pancake), a transmembrane domain (two blue cylinders), and an intracellular domain (two strings).
(B) When ATP binds to the extracellular domain, the ATP receptor changes its shape so that the three subunits should form a pore in their center. The pore is a path for ions and other small molecules that transmit signals into cells.
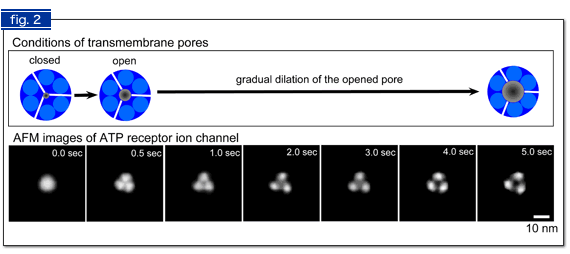
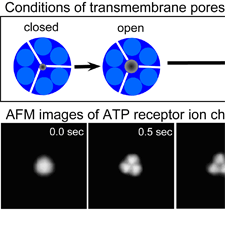
Fig. 2 AFM images of ATP-induced structural changes in ATP receptor.
At the moment that ATP was added (0 sec), a single ATP receptor was observed as a round shape (corresponding to Fig. 1B left). 0.5 sec later, three subunits were separately observed, in addition to a small pore in their center (Fig. 1B right). When ATP stimulus continued under Ca2+ free conditions, we found that a bigger pore, which allows bigger molecules to pass, was formed (4 - 5 sec).










